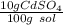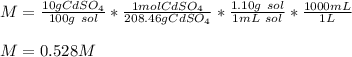Chemistry, 07.01.2021 18:30, arieltaylor3924
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol) by mass? The density of the solution is 1.10 g/mL.
a. 0.528 M
b. 0.436 M
c. 0.479 M
d. 0.048 M
e. 22.9 M

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2


Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
Do you know the correct answer?
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol)...
Questions in other subjects:

Mathematics, 24.11.2020 01:00



Business, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Arts, 24.11.2020 01:00

History, 24.11.2020 01:00


English, 24.11.2020 01:00