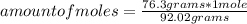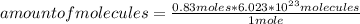Chemistry, 07.01.2021 18:30, orangeicecream
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^25 N2O4 molecules
b. 5.54 × 10^25 N2O4 molecules
c. 7.26 × 10^23 N2O4 molecules
d. 1.38 × 10^24 N2O4 molecules
e. 4.99 × 10^23 N2O4 molecules

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:40, ohgeezy
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1


Chemistry, 23.06.2019 12:30, ritahastie7533
Atriple covalent bond involves two atoms sharing three pairs of electrons. true false
Answers: 2
Do you know the correct answer?
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^2...
Questions in other subjects:



English, 22.12.2020 09:30

English, 22.12.2020 09:30



English, 22.12.2020 09:30



English, 22.12.2020 09:30