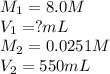Chemistry, 07.01.2021 18:00, aubreyfoster
g A chemist must prepare of hydrochloric acid solution with a pH of at . He will do this in three steps: Fill a volumetric flask about halfway with distilled water. Measure out a small volume of concentrated () stock hydrochloric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
Do you know the correct answer?
g A chemist must prepare of hydrochloric acid solution with a pH of at . He will do this in three st...
Questions in other subjects:

English, 02.11.2020 19:00

English, 02.11.2020 19:00



Computers and Technology, 02.11.2020 19:00




Mathematics, 02.11.2020 19:00

Mathematics, 02.11.2020 19:00


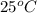 . He will do this in three steps: Fill a 550.0 mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (8.0M) stock hydrochloric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.
. He will do this in three steps: Fill a 550.0 mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (8.0M) stock hydrochloric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.![pH=-\log [H^+]](/tpl/images/1019/0049/37e81.png)
![1.60=-\log [H^+]](/tpl/images/1019/0049/bdcbd.png)
![[H^+]=antilog (-1.60)](/tpl/images/1019/0049/c0db8.png)
![[H^+]=0.0251M](/tpl/images/1019/0049/a5993.png)
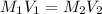
 = molarity and volume of concentrated acid solution
= molarity and volume of concentrated acid solution = molarity and volume of diluted acid solution
= molarity and volume of diluted acid solution