Iii) Pentane burns according to the following equation:
C5H12(g) +
802(9)
5CO2(9)
...

Chemistry, 07.01.2021 14:30, MalikaJones
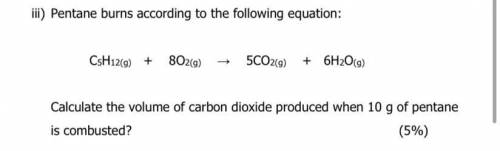
Iii) Pentane burns according to the following equation:
C5H12(g) +
802(9)
5CO2(9)
+ 6H2O(g)
Calculate the volume of carbon dioxide produced when 10 g of pentane
is combusted?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


Do you know the correct answer?
Questions in other subjects:

Physics, 12.12.2020 18:30

Mathematics, 12.12.2020 18:30

Mathematics, 12.12.2020 18:30



Health, 12.12.2020 18:30


Biology, 12.12.2020 18:30

Mathematics, 12.12.2020 18:30






