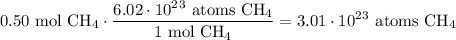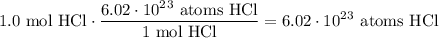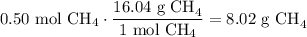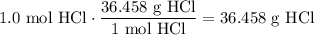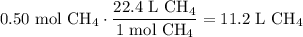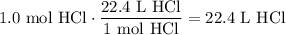Chemistry, 07.01.2021 14:00, truesarah111
Given the following two quantities: 0.50 mol of CH4 and 1.0 mol of HCl,
a. Which has more atoms?
b. Which has more molecules?
c. Which has the greater mass?
d. Which has the greater volume at the same temperature and pressure (both are gases)?

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
Do you know the correct answer?
Given the following two quantities: 0.50 mol of CH4 and 1.0 mol of HCl,
a. Which has more atoms?
Questions in other subjects:


Geography, 29.08.2019 02:30


Mathematics, 29.08.2019 02:30

English, 29.08.2019 02:30

Physics, 29.08.2019 02:30


English, 29.08.2019 02:30