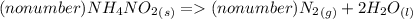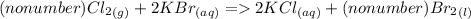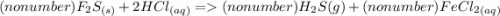ANSWER ASAP
1. NH₄NO₂(s) → N₂(g) + H₂O(l)
1a. Write the number (if any) that should go i...

ANSWER ASAP
1. NH₄NO₂(s) → N₂(g) + H₂O(l)
1a. Write the number (if any) that should go in front of NH₄NO₂(s), N₂(g), and H₂O(l) in order with commas in between. If there shouldn't be a number, write "no number".
1b. What type of reaction is represented in this equation?
2. Cl₂(g) + KBr(aq) → KCl (aq) + Br₂(l)
2a. Write the number (if any) that should go in front of each compound in order with commas in between. If there shouldn't be a number, write "no number".
2b. What type of reaction is represented in this equation?
3. FeS(s) + HCl(aq) → H₂S(g) + FeCl₂ (aq)
3a. Write the number (if any) that should go in front of each compound in order with commas in between. If there shouldn't be a number, write "no number".
3b. What type of reaction is represented in this equation?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Do you know the correct answer?
Questions in other subjects: