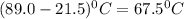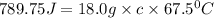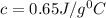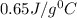Chemistry, 06.01.2021 20:20, koryhudson8124
An 18.0 g piece of an unidentified metal was heated from 21.5 °C to 89.0 °C. If 789.75 J of heat energy was absorbed by the metal in the heating process, what was the identity of the metal?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, sairaanwar67
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 21.06.2019 19:00, lazavionadams81
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
Do you know the correct answer?
An 18.0 g piece of an unidentified metal was heated from 21.5 °C to 89.0 °C. If 789.75 J of heat ene...
Questions in other subjects:




Mathematics, 27.03.2020 16:01

Mathematics, 27.03.2020 16:01

Geography, 27.03.2020 16:01


Mathematics, 27.03.2020 16:01


Mathematics, 27.03.2020 16:01


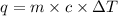
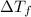 = final temperature - initial temperature =
= final temperature - initial temperature =