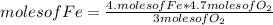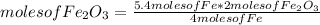Chemistry, 05.01.2021 16:30, gennhill14
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that can be produced? What is the limiting reactant?
a
3.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
b
2.7 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.
c
7.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
d
10.8 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3
Do you know the correct answer?
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that c...
Questions in other subjects:

Mathematics, 07.05.2021 01:00

History, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

English, 07.05.2021 01:00



English, 07.05.2021 01:00