
Chemistry, 03.01.2021 04:50, bossboybaker
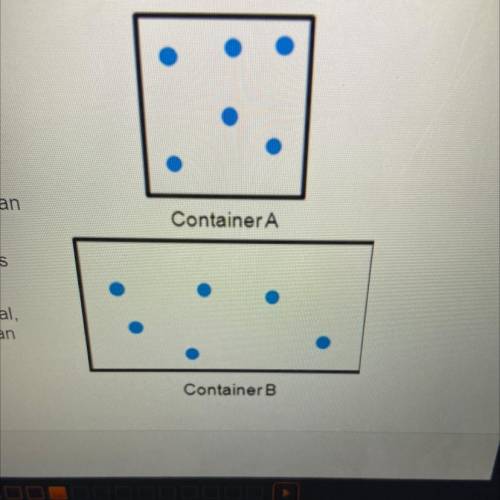
The diagrams to the right show the distribution
and arrangement of gas particles in two different
containers. According to kinetic-molecular theory,
which of the following statements is true? Check
all that apply.
If the temperatures of both containers are
equal, container A has greater pressure than
container B.
ContainerA
U
If the volume of container A decreased, its
pressure would decrease.
If the pressure in both containers is equal,
container A has a lower temperature than
container B.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:50, mia36492
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table. state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
Do you know the correct answer?
The diagrams to the right show the distribution
and arrangement of gas particles in two different
Questions in other subjects:


Mathematics, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01

Advanced Placement (AP), 05.10.2020 16:01

Health, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01



History, 05.10.2020 16:01






