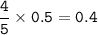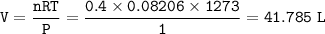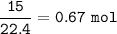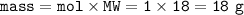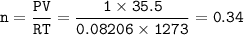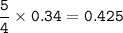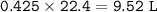Catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (H...

Catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (HNO3) on a commercial scale.
The products are produced at 1000°C (1273 K) and at at-
mospheric pressure.
4 NH; (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (1)
a. What volume of NO is produced in the reaction vessel
by the reaction of 0.500 mol O2?
b. What mass of H2O is produced by the reaction of 15.0 L
of NH3?
c. How many liters of O, must react to produce 35.5 L of
NO?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1


Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Mathematics, 19.08.2020 04:01


Mathematics, 19.08.2020 04:01


Social Studies, 19.08.2020 04:01

Mathematics, 19.08.2020 04:01