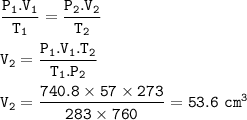Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kristineford198
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3



Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
Do you know the correct answer?
A certain mass of hydrogen gas collected over water at 10°C and 750mmHg pressure has a volume of 57c...
Questions in other subjects:









Mathematics, 05.05.2020 04:01

Mathematics, 05.05.2020 04:01