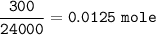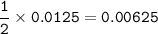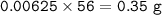4) 300cm of Hydrogen chloride gas were passed over 7.0g of heated iron fillings until there was no further
change. The reaction vessel was then allowed to cool to room temperature. Use the equation below to
determine the mass of iron that remained at the end of the experiment (molar gas volume = 24000cm"; Fe=56).
Fe(s) + 2HCl(g) → FeCl2(s) + H2(g)
(3 mks)
50
3 of IM colution calcium chloride (Avogadro's

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
Do you know the correct answer?
4) 300cm of Hydrogen chloride gas were passed over 7.0g of heated iron fillings until there was no f...
Questions in other subjects:



Computers and Technology, 18.03.2020 00:58

Mathematics, 18.03.2020 00:58


Computers and Technology, 18.03.2020 00:59

Social Studies, 18.03.2020 00:59


Mathematics, 18.03.2020 00:59

History, 18.03.2020 00:59