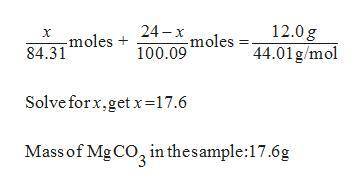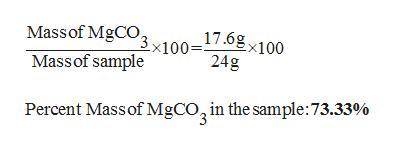30. A solid sample contains only CaCO3, and MgCO3.
To completely react the CaCO3 and MgCO3
pre...

Chemistry, 01.01.2021 20:00, joleiswan9919
30. A solid sample contains only CaCO3, and MgCO3.
To completely react the CaCO3 and MgCO3
present in the sample. 42.00 cm of 0.088 M
HCI were required. The anhydrous chloride salts
from the reaction, obtained by evaporation of
the filtrate weighed 0.19 g. The mass of CaCO3
present in the solid sample is (C = 12,0 = 16,
Mg = 24, Ca = 40, CI = 35.5 )
(1) 0.05 g.
(2) 0.07 g
(3) 0.09 g
(4) 0.11g
(5) 0.12 g

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

History, 27.12.2019 02:31




Mathematics, 27.12.2019 02:31

History, 27.12.2019 02:31

History, 27.12.2019 02:31

English, 27.12.2019 02:31

Social Studies, 27.12.2019 02:31