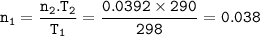Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3


Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
Do you know the correct answer?
3.2 g of a gas at occupy the same volume as 0.6266 g of CH4 at 17°C at constant pressure
Find the m...
Questions in other subjects:


Mathematics, 30.07.2019 03:10



Mathematics, 30.07.2019 03:10

Mathematics, 30.07.2019 03:10

Mathematics, 30.07.2019 03:10

English, 30.07.2019 03:10

Mathematics, 30.07.2019 03:10