temperature will change from

Chemistry, 31.12.2020 02:20, katelynalivia
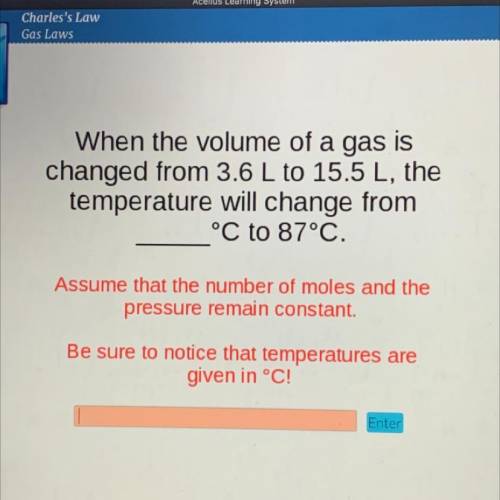
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
°C to 87°C.
Assume that the number of moles and the
pressure remain constant.
Be sure to notice that temperatures are
given in °C!

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 08:00, colbygreen6189
Identify the decay mode particle emitted from the th 234
Answers: 1
Do you know the correct answer?
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
temperature will change from
Questions in other subjects:


Mathematics, 23.08.2019 19:50

History, 23.08.2019 19:50

Mathematics, 23.08.2019 19:50




English, 23.08.2019 19:50


Mathematics, 23.08.2019 19:50






