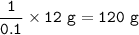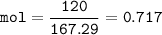Chemistry, 29.12.2020 23:20, dmurdock1973
What is the concentration in mol L-l of a 12% solution of tetrahydrate magnesium chloride (MgCl2)?
Mg = 24.31 g mol-1
Cl = 35.45 g mol-1
H = 1.01 g mol-1
O = 16.00 g mol-1

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3
Do you know the correct answer?
What is the concentration in mol L-l of a 12% solution of tetrahydrate magnesium chloride (MgCl2)?...
Questions in other subjects:


English, 27.03.2020 05:33






Mathematics, 27.03.2020 05:33

Mathematics, 27.03.2020 05:33

Mathematics, 27.03.2020 05:33