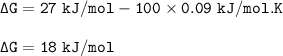Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
Do you know the correct answer?
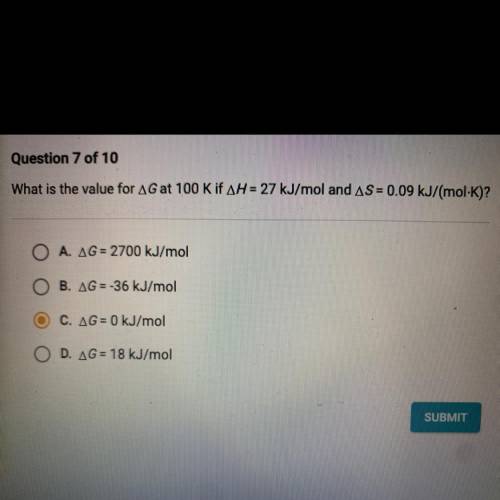
What is the value for AG at 100 K if AH = 27 kJ/mol and AS = 0.09 kJ/mol. K)?
O A. AG = 2700 kJ/mol...
Questions in other subjects:







Engineering, 22.01.2020 01:31