
Chemistry, 29.12.2020 22:00, jessicaaaamartin
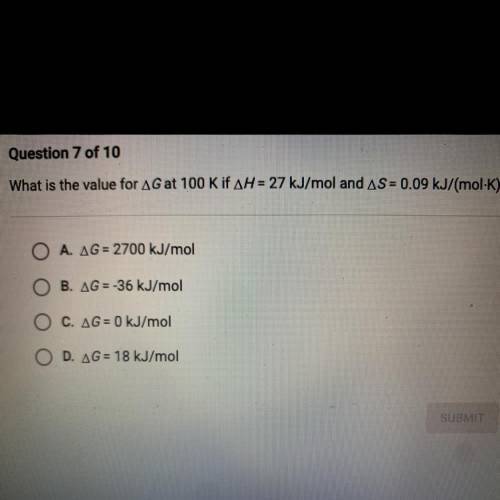
What is the value for AG at 100 K if AH = 27 kJ/mol and AS = 0.09 kJ/(mol-K)?
A. AG= 2700 kJ/mol
B. AG = -36 kJ/mol
C. AG = 0 kJ/mol
D. AG = 18 kJ/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 08:40, Riplilpeep
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1

Chemistry, 23.06.2019 09:00, student0724
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
Do you know the correct answer?
What is the value for AG at 100 K if AH = 27 kJ/mol and AS = 0.09 kJ/(mol-K)?
A. AG= 2700 kJ/mol
Questions in other subjects:

Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32


English, 22.01.2020 06:32





Mathematics, 22.01.2020 06:32






