Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1
Do you know the correct answer?
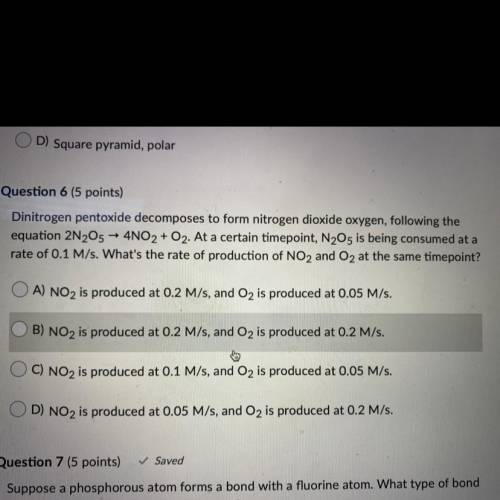
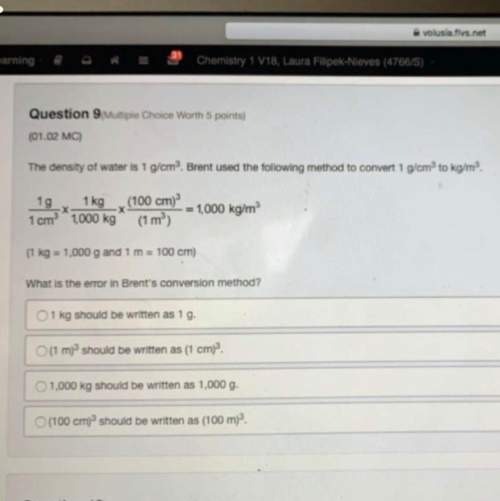
dinitrogen pentoxide decomposes to form nitrogen dioxide oxygen, following the equation 2N2O5 ->...
Questions in other subjects:




English, 24.09.2019 17:30


Biology, 24.09.2019 17:30


Mathematics, 24.09.2019 17:30