

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, gracelanghorn
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
Do you know the correct answer?
1.72 moles of NOCI were placed in a 2.50 L reaction chamber
at 427°C. After equilibrium was reached...
Questions in other subjects:













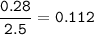
![\tt Kc=\dfrac{[NO]^2[Cl_2]}{[NOCl]^2}\\\\Kc=\dfrac{0.224^2\times 0.112}{0.464^2}=0.026](/tpl/images/1007/8914/ad1b4.png)




