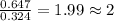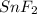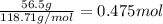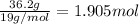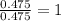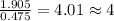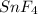Chemistry, 05.10.2019 16:20, devinluck100
Tin reacts with fluorine to form two different compounds, a and b. compound a contains 38.5 g of tin for each 12.3 g of fluorine. compound b contains 56.5 g of tin for each 36.2 g of fluorine. what is the lowest whole-number mass ratio of tin that combines with a given mass of fluorine?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Do you know the correct answer?
Tin reacts with fluorine to form two different compounds, a and b. compound a contains 38.5 g of tin...
Questions in other subjects:

English, 23.08.2019 18:10

English, 23.08.2019 18:10


Mathematics, 23.08.2019 18:10

English, 23.08.2019 18:10


History, 23.08.2019 18:10


Computers and Technology, 23.08.2019 18:10