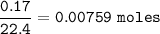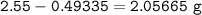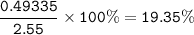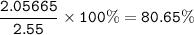Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Do you know the correct answer?
2.55 gram of an alloy of silver and zinc on treatment with excess dilute hydrochloric acid gave 170...
Questions in other subjects:


Mathematics, 02.04.2021 18:40

Social Studies, 02.04.2021 18:40


Chemistry, 02.04.2021 18:40


Mathematics, 02.04.2021 18:40


Mathematics, 02.04.2021 18:40