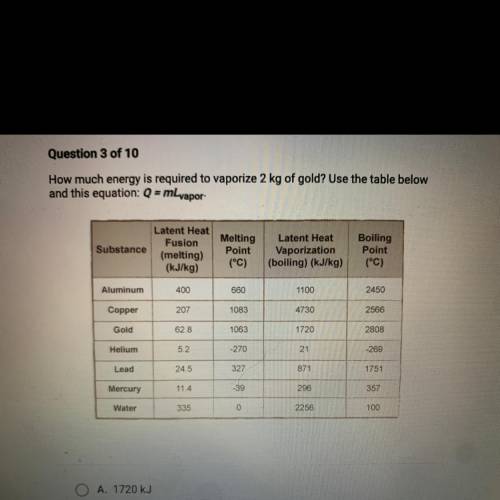
A. 1720 kJ
B. 125.6 kJ
C. 3440 kJ
D. 4730 kJ
...

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Social Studies, 01.09.2019 21:30

Social Studies, 01.09.2019 21:30


English, 01.09.2019 21:30


Biology, 01.09.2019 21:30


Mathematics, 01.09.2019 21:30


Mathematics, 01.09.2019 21:30