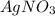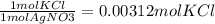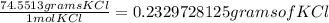Chemistry, 23.12.2020 17:40, biaxialpower789
11. Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride:
KCl(aq) + AgNO3(aq) → AgCl(s) + KNO3(aq)
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion. How many grams of solid KCl must be added to 25.0 mL of 0.125 M AgNO3 solution to completely precipitate the silver?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 23.06.2019 00:00, scottykinkade7860
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
Do you know the correct answer?
11. Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride:
KCl...
Questions in other subjects:



Mathematics, 30.05.2020 13:57

Law, 30.05.2020 13:57

Mathematics, 30.05.2020 13:57






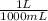 ) = 0.025 L
) = 0.025 L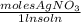 ) = 0.003125 moles
) = 0.003125 moles