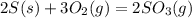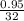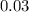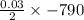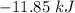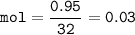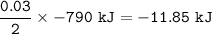Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
Do you know the correct answer?
The value of ΔH° for the reaction below is -790 kJ. The enthalpy change accompanying the reaction of...
Questions in other subjects:

Mathematics, 02.02.2021 03:00

Social Studies, 02.02.2021 03:00



Mathematics, 02.02.2021 03:00


Biology, 02.02.2021 03:00

Mathematics, 02.02.2021 03:00

Mathematics, 02.02.2021 03:00