
1. The gases SO2, O2 and SO3 are allowed to reach equilibrium at a constant temperature. The equilibrium constant for the reaction
2SO2(g) + O2(g) -> 2S03(g)
is 1.6 x 104 atm-1
a) Calculate the value of Kp for the reaction
SO2(g) + ½O2(g) -> SO3(g)
(b) The equilibrium constant for the dissociation of Pcl5(g) to form PCl3(g) and Cl2(g) is
0.04 at 250°C. An equilibrium mixture contains 0.20 mol PC13 and 0.12 mol Cl, in a
4000 cm container.
i) Write the chemical equation.
ii) Calculate the concentration of PCIs in this container.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, paolaviviana
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
Do you know the correct answer?
1. The gases SO2, O2 and SO3 are allowed to reach equilibrium at a constant temperature. The equilib...
Questions in other subjects:

Mathematics, 17.11.2020 18:30



Health, 17.11.2020 18:30

English, 17.11.2020 18:30


Mathematics, 17.11.2020 18:30


Mathematics, 17.11.2020 18:30

Mathematics, 17.11.2020 18:30


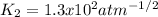
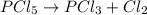
![[PCl_5]=0.0375M](/tpl/images/1006/3442/49c8a.png)
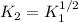
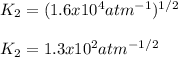
![K=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/1006/3442/bc353.png)
![[PCl_5]=\frac{[PCl_3][Cl_2]}{K}\\](/tpl/images/1006/3442/edc14.png)
![[PCl_5]=\frac{\frac{0.20mol}{4L} *\frac{0.12mol}{4L} }{0.04}](/tpl/images/1006/3442/09a24.png)




