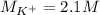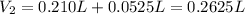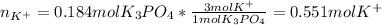Chemistry, 23.12.2020 04:30, neekobecky599
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water to form a 0.700 M K3PO, solution. Calculate the molarity of potassium ions, K in the solution.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3


Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2
Do you know the correct answer?
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water
t...
Questions in other subjects:

Mathematics, 16.10.2020 19:01

Biology, 16.10.2020 19:01




English, 16.10.2020 19:01

English, 16.10.2020 19:01


Chemistry, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01