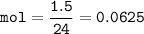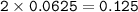Chemistry, 22.12.2020 19:10, Flowershere121
Sodium hydrogen carbonate, on heating, produces sodium carbonate, water and carbon dioxide.
A recipe for chocolate chip cookies requires 1.5 dm' of carbon dioxide.
Calculate the mass of sodium hydrogen carbonate that should be used at R. T.P.
INa = 23; H = 1: C = 12; O = 16.]
[Note that 1 mole of gas occupies a volume of 24,000 cm at room temperature and pressure (RTP)]

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Do you know the correct answer?
Sodium hydrogen carbonate, on heating, produces sodium carbonate, water and carbon dioxide.
A recip...
Questions in other subjects:

Mathematics, 19.05.2021 19:10

Chemistry, 19.05.2021 19:10



English, 19.05.2021 19:10