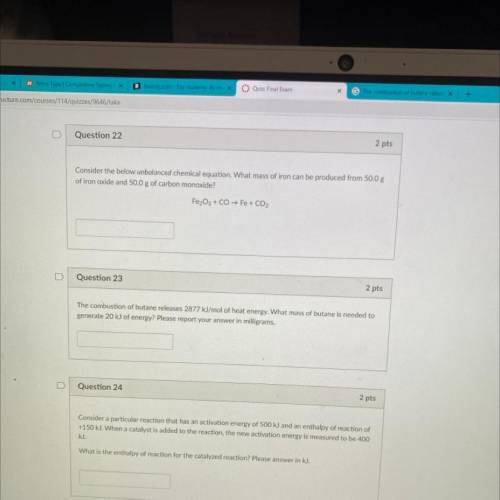
Question 22
2 pts
Consider the below unbalanced chemical equation. What mass of iron can be p...

Chemistry, 22.12.2020 09:10, nattalliiee67
Question 22
2 pts
Consider the below unbalanced chemical equation. What mass of iron can be produced from 50.0 g
of iron oxide and 50.0 g of carbon monoxide?
Fe2O3 + CO → Fe + CO2
Question 23
2 pts
The combustion of butane releases 2877 kJ/mol of heat energy. What mass of butane is needed to
generate 20 kJ of energy? Please report your answer in milligrams.
Question 24
2 pts
Consider a particular reaction that has an activation energy of 500 kJ and an enthalpy of reaction of
+150 kJ. When a catalyst is added to the reaction, the new activation energy is measured to be 400
kJ.
What is the enthalpy of reaction for the catalyzed reaction? Please answer in kJ.
O

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1



Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 04.02.2020 17:43


English, 04.02.2020 17:43

Mathematics, 04.02.2020 17:43

Computers and Technology, 04.02.2020 17:43




English, 04.02.2020 17:43






