
Chemistry, 22.12.2020 06:40, 91miketaylor
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57.93 amu respectively. What is the atomic mass of these two isotopes? Explain why
the average mass is closer to actual amu of 58.69 for nickel.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
Do you know the correct answer?
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57...
Questions in other subjects:

English, 20.04.2021 16:00



Mathematics, 20.04.2021 16:00

Business, 20.04.2021 16:00



Mathematics, 20.04.2021 16:00

Mathematics, 20.04.2021 16:00


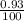


![A=\sum[(63.93)\times \frac{0.93}{100})+(57.93)\times \frac{68.08}{100}]]](/tpl/images/1005/1681/01772.png)






