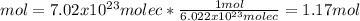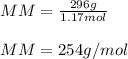Chemistry, 22.12.2020 04:20, dchannakhone84
7.02 x 10^23 molecules of X2, a ssubstance consisting of diatomic molecules, has a mass of 296 grams. Determine the atomic weight (mass of a mole of atoms) of element X.
a. 254 g/mol.
b. 296 g/mol.
c. 148 g/mol.
d. 127 g/mol.
e. 507 g/mol.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 00:20, cmflores3245
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
Do you know the correct answer?
7.02 x 10^23 molecules of X2, a ssubstance consisting of diatomic molecules, has a mass of 296 grams...
Questions in other subjects: