Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2


Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
Do you know the correct answer?
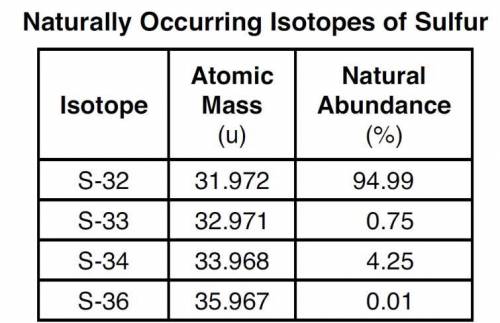
The four naturally occurring isotopes of sulfur are S-32, S-33, S-34, and S-36. The table below show...
Questions in other subjects:





Mathematics, 03.09.2021 20:00


Mathematics, 03.09.2021 20:00


Health, 03.09.2021 20:00

Mathematics, 03.09.2021 20:00