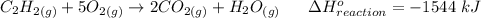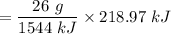Chemistry, 21.12.2020 18:10, alexantkoviak13
The combustion of ethyne, shown below unbalance, produces heat which can be used to weld metals:
C2H2 (g) +502 (g) →2CO: (g) +H20 (g) AH reaction= -1544kJ
How much ethyne gas (in g) would you need to react with excess oxygen according to this reaction in order to raise the temperature of 325 g of high carbon steel from 165'C to its melting point, 1540 C? The heat capacity of high carbon steel is 0.490 J/g'C. (Assume a complete reaction and that all heat is transferred from the reaction to the metal with no loss.)
a. 7.37g
b. 1.84 g
c. 4.13 g
d 3.69 g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
Do you know the correct answer?
The combustion of ethyne, shown below unbalance, produces heat which can be used to weld metals:
C2...
Questions in other subjects:



Social Studies, 24.09.2021 14:00

History, 24.09.2021 14:00

History, 24.09.2021 14:00


English, 24.09.2021 14:00


Health, 24.09.2021 14:00


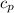 = 0.490 J/g°C
= 0.490 J/g°C