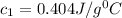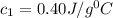When 100.0 g block of a metal at 600 oC is plunged into 100.0 g of water (specific heat capacity 4.2 J/g. oC) at 30 oC, the final temperature of both the metal and the water is 80 oC. If no heat is lost to the surroundings, what is the specific heat capacity of the metal in J/g. oC? Give your answer to 2 significant figures.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3


Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2
Do you know the correct answer?
When 100.0 g block of a metal at 600 oC is plunged into 100.0 g of water (specific heat capacity 4.2...
Questions in other subjects:


English, 19.05.2021 16:10

Arts, 19.05.2021 16:10



Arts, 19.05.2021 16:10




Mathematics, 19.05.2021 16:10


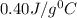
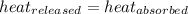
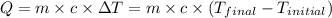
![-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1004/1883/92a72.png) .................(1)
.................(1)
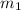 = mass of metal = 100.0 g
= mass of metal = 100.0 g
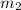 = mass of water = 100.0 g
= mass of water = 100.0 g
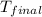 = final temperature =
= final temperature = 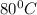
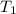 = temperature of metal =
= temperature of metal = 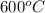
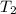 = temperature of water =
= temperature of water = 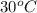
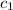 = specific heat of metal = ?
= specific heat of metal = ?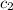 = specific heat of water=
= specific heat of water= 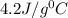
![-[100.0\times c_1\times (80-600)]=[100.0\times 4.2\times (80-30)]](/tpl/images/1004/1883/4e506.png)