
Chemistry, 21.12.2020 17:50, krishawnnn
A voltaic cell is constructed that is based on the following reaction:
Sn2+(aq)+Pb(s)→Sn(s)+Pb2+(aq).
If the concentration of Sn2+ in the cathode compartment is 1.00 M and the cell generates an emf of 0.16 V , what is the concentration of Pb2+ in the anode compartment?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Do you know the correct answer?
A voltaic cell is constructed that is based on the following reaction:
Sn2+(aq)+Pb(s)→Sn(s)+Pb2+(a...
Questions in other subjects:

SAT, 06.12.2021 20:40



Mathematics, 06.12.2021 20:40



Biology, 06.12.2021 20:40

Social Studies, 06.12.2021 20:40

Mathematics, 06.12.2021 20:40


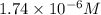

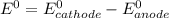
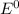 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Sn^{2+}/Sn]}=-0.14V](/tpl/images/1004/1474/81a51.png)
![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/1004/1474/82211.png)
![E^0=E^0_{[Sn^{2+}/Sn]}- E^0_{[Pb^{2+}/Pb]}](/tpl/images/1004/1474/cb409.png)
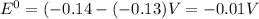
![E=E^0-\frac{0.059}{n}\log\frac{[Pb^{2+}]}{[Sn^{2+}]}](/tpl/images/1004/1474/ec777.png)
![0.16=(-0.01)-\frac{0.059}{2}\log\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/70ccb.png)
![0.17=-0.0295\log\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/5042d.png)
![-5.76=\log\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/a4415.png)
![1.74\times 10^{-6}=\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/3d2ba.png)
![[Pb^{2+}]=1.74\times 10^{-6}](/tpl/images/1004/1474/109b6.png)




