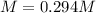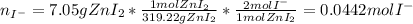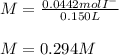Chemistry, 21.12.2020 17:30, aidenbender06
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbonate. Calculate the final molarity of iodide anion in the solution. You can assume the volume of the solution doesn't change when the zinc iodide is dissolved in it. Round your answer to 3 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
Do you know the correct answer?
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbo...
Questions in other subjects:


Biology, 16.12.2021 18:00

Health, 16.12.2021 18:00

Mathematics, 16.12.2021 18:00

Mathematics, 16.12.2021 18:00





Chemistry, 16.12.2021 18:00