
Chemistry, 21.12.2020 16:50, Krazyyykiddd
Dilute hydrochloric acid was titrated with sodium carbonate solution. • 10.0 cm3 of 0.100 mol / dm3 hydrochloric acid were placed in a conical flask. • A few drops of methyl orange indicator were added to the dilute hydrochloric acid. • The mixture was titrated with sodium carbonate solution. • 16.2 cm3 of sodium carbonate solution were required to react completely with the acid. What colour would the methyl orange indicator be in the hydrochloric acid? Calculate how many moles of hydrochloric acid were used. Use your answer to (b)(ii) and the equation for the reaction to calculate the number of moles of sodium carbonate that reacted.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 23.06.2019 01:00, aliviadushane
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
Do you know the correct answer?
Dilute hydrochloric acid was titrated with sodium carbonate solution. • 10.0 cm3 of 0.100 mol / dm3...
Questions in other subjects:

Law, 31.12.2020 09:00


English, 31.12.2020 09:00

Mathematics, 31.12.2020 09:00

Mathematics, 31.12.2020 09:00






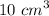 of a 0.100
of a 0.100 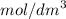 of hydrocholric acid is used.
of hydrocholric acid is used.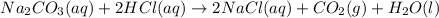
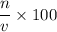
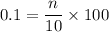
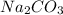 , two moles of HCl acid is used.
, two moles of HCl acid is used.




