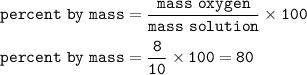Chemistry, 19.12.2020 22:20, 1xXJOKERXx3
The percent by mass is the ratio of the mass of each element to the total mass of the compound expressed as a percentage. Attached is the formula. What is the percent by mass of Oxygen in 10 grams of H20 given the mass by analysis of oxygen in that sample is 8 grams. (Answer should be given with a %. i. e. if the answer were 23% you answer in the textbox would be 23%)
Percent by mass = mass of solute mass of solution x 100
help me please:((
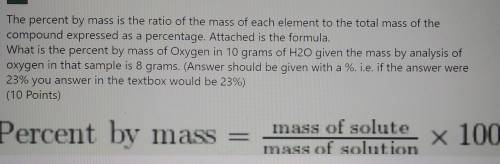

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, penny3109
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
Do you know the correct answer?
The percent by mass is the ratio of the mass of each element to the total mass of the compound expre...
Questions in other subjects:


Mathematics, 28.09.2019 10:30



English, 28.09.2019 10:30

Mathematics, 28.09.2019 10:30



Mathematics, 28.09.2019 10:30

Mathematics, 28.09.2019 10:30