Chemistry, 18.12.2020 22:20, blackwelle72
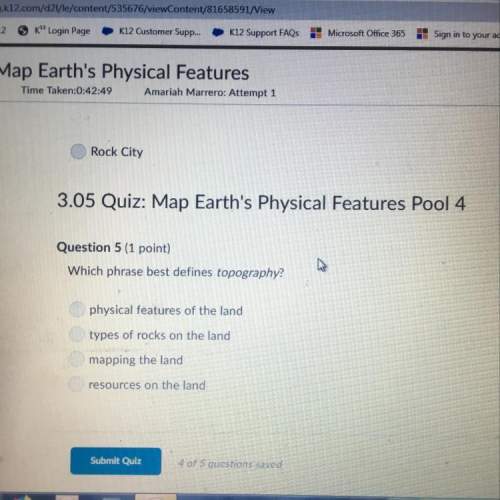
17. The formula for iron (1) cyanide is: *
(1 Point)
O FACN
Fec
O FeCN
Fe2CN3
O Feat

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, lizzyhearts
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 01:30, Elliendc7939
List and describe the neurological effects of the vocs and other air pollutants, as described by dr. theo colborn
Answers: 2
Do you know the correct answer?
17. The formula for iron (1) cyanide is: *
(1 Point)
O FACN
Fec
O FeCN
Fe2C...
O FACN
Fec
O FeCN
Fe2C...
Questions in other subjects:




Mathematics, 20.10.2020 21:01


English, 20.10.2020 21:01


Mathematics, 20.10.2020 21:01


English, 20.10.2020 21:01