GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Ques...

Chemistry, 18.12.2020 04:00, sabahtramirez01
GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Question :
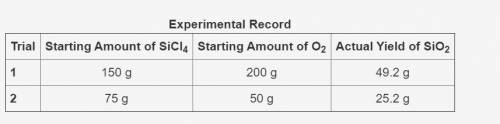
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
The table shows an experimental record for the above reaction.
-> THE TABLE IS IN THE IMAGE <-
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 2
Other questions on the subject: Chemistry



Do you know the correct answer?
Questions in other subjects:




Spanish, 29.08.2020 21:01




Chemistry, 29.08.2020 21:01







