
Chemistry, 18.12.2020 01:00, reneebrown017
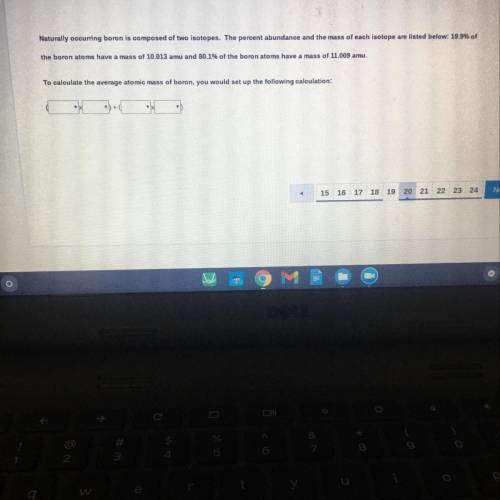
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each isotope are listed below: 19.9% of
ne boron atoms have a mass of 10.013 amu and 80.1% of the boron atoms have a mass of 11.009 amu.
calculate the average atomic mass of boron, you would set up the following calculation:
What

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1
Do you know the correct answer?
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each is...
Questions in other subjects:

Chemistry, 19.11.2020 18:20

Biology, 19.11.2020 18:20


Social Studies, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20









