
Chemistry, 17.12.2020 20:00, mikurrjurdan
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and delta H rxn given above, which of the following can best be used to justify that the reaction is thermodynamically favorable at 298 K and constant pressure?
N2 (g) + 3H2 (g) --> 2NH3 (g)
K= 5.6 × 10^5 at 298 K
delta H rxn= -91.8 kj/mol
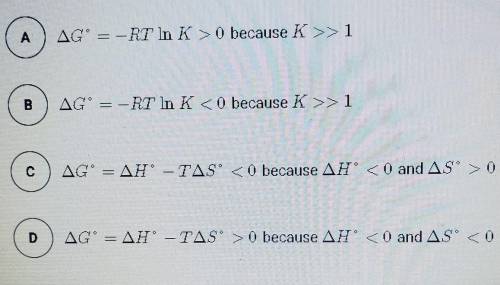

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1


Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
Do you know the correct answer?
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and...
Questions in other subjects:

Mathematics, 09.04.2021 22:40




French, 09.04.2021 22:40


Mathematics, 09.04.2021 22:40

English, 09.04.2021 22:40








