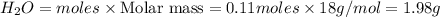Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
Do you know the correct answer?
Use the balanced equation given below to solve the problem that follows: Calculate the mass in grams...
Questions in other subjects:


English, 20.04.2021 06:20

Mathematics, 20.04.2021 06:20

Mathematics, 20.04.2021 06:20

Mathematics, 20.04.2021 06:20


History, 20.04.2021 06:20


Biology, 20.04.2021 06:20


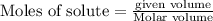
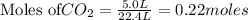
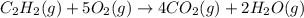
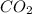 will produce = 2 moles of
will produce = 2 moles of 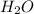
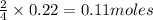 of
of