Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3


Chemistry, 23.06.2019 01:20, michellectucker1982
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 07:20, msladycie8831
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
Do you know the correct answer?
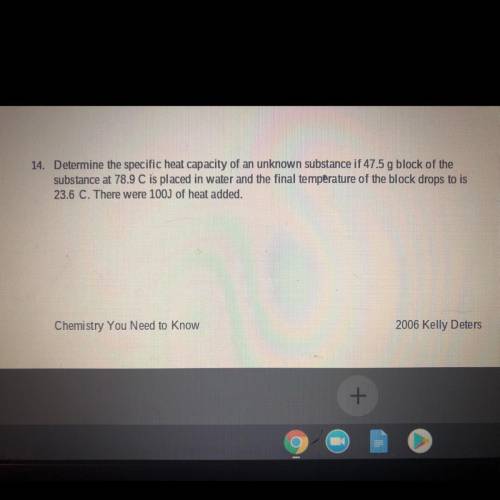
Determine the specific heat capacity of an unknown substance if 47.5 g block of the substance at 78....
Questions in other subjects:

Chemistry, 05.11.2020 21:40


English, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40



History, 05.11.2020 21:40

English, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40