
Chemistry, 17.12.2020 08:10, ronniethefun
SOMEONE PLEASE HELP I DON'T GET THIS AT ALL
Modeling Energy Changes Student Guide on Edge
Step 3: Determine the amount of energy change in the reaction.
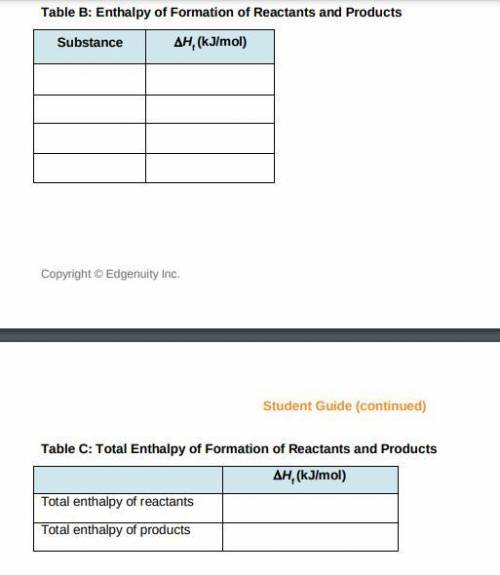
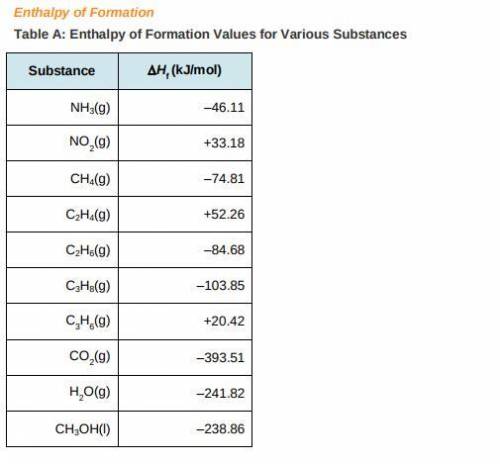
a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the enthalpy of formation (DeltaHt) for each reactant and each product. Record these values along with the reactants and products in Table B of the Student Worksheet.
b) Determine the total enthalpy of the reactants and the total enthalpy of the products Record these values in Table C of the Student Worksheet.
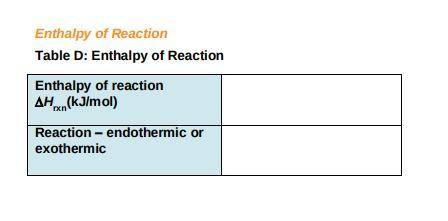
c) Use the following formula to find the net change in enthalpy for the reaction and to determine whether the reaction is endothermic is endothermic or exothermic.
ΔHrxn= ∑ (Δ Hf, products)- ∑ (ΔHf, reactants)
Record your answers in Table D.
Step 4: Model the energy change in the reaction.
a) Create an energy graph that illustrates the energy change in the reaction.
b)Construct your graph on a blank sheet of paper. Be sure to label the axes, provide a title, and identify the reactants and product on the graph.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1
Do you know the correct answer?
SOMEONE PLEASE HELP I DON'T GET THIS AT ALL
Modeling Energy Changes Student Guide on Edge
Questions in other subjects:


Mathematics, 08.04.2020 20:51

English, 08.04.2020 20:51













