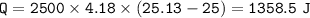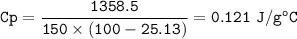Chemistry, 16.12.2020 08:10, kierafisher05
The murder weapon weighs 150 grams. The initial temperature of the weapon before being tested was 100 oC. After placing the weapon in the water, the temperature dropped to 25.13 oC. Find the specific heat capacity of the unknown metal by first calculating the heat energy gained by the water.
Water
Mass = 2500 g
Initial temperature = 25 o C Final temperature = 25.13 oC
Equations
q = m C p Δ T C p = q / (mΔT)
1. How much heat energy (q) did the water gain?
2. What is the specific heat capacity (C p) of the unknown metal?
3. Compare the heat capacity you calculated with the one below, which one matches?
Specific heat capacity of metal (J / g or C)
Silver 0.235
Gold 0.129 Lead 0.121
Copper
4. What metal was the murder weapon made of?
0.385

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2


Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
Do you know the correct answer?
The murder weapon weighs 150 grams. The initial temperature of the weapon before being tested was 10...
Questions in other subjects:

Mathematics, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00

English, 22.04.2021 02:00

English, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00