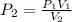The pressure inside of a sealed syringe of hydrogen gas is 80.0KPa with a volume of 250 Liters. What is the pressure inside
of the syringe after the plunger is pulled back far enough to make final volume of the syringe 0.750 Liters? (assume the
temperature and amount of gas in the container are held constant)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1


Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1
Do you know the correct answer?
The pressure inside of a sealed syringe of hydrogen gas is 80.0KPa with a volume of 250 Liters. What...
Questions in other subjects:

Social Studies, 18.05.2021 18:40


Mathematics, 18.05.2021 18:40

Biology, 18.05.2021 18:40





Mathematics, 18.05.2021 18:40