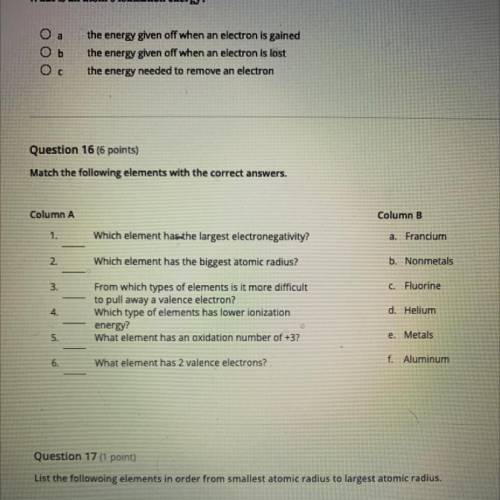
Question 16 (6 points)
Match the following elements with the correct answers.
Column A
...

Chemistry, 16.12.2020 01:40, asdfghhk9805
Question 16 (6 points)
Match the following elements with the correct answers.
Column A
Column B
a Francium
1.
Which element has the largest electronegativity?
2
Which element has the biggest atomic radius?
b. Nonmetals
3.
C Fluorine
4
From which types of elements is it more difficult
to pull away a valence electron?
Which type of elements has lower ionization
energy?
What element has an oxidation number of +3?
d. Helium
5.
e. Metals
6.
What element has 2 valence electrons?
f. Aluminum

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1


Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Advanced Placement (AP), 24.02.2021 22:40

Health, 24.02.2021 22:40


Biology, 24.02.2021 22:40

Mathematics, 24.02.2021 22:40

World Languages, 24.02.2021 22:40









