

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1


Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
Do you know the correct answer?
A sample of helium gas at 841 mmHg and 14.7°C is heated to 84.7°C at constant volume. Calculate its...
Questions in other subjects:


History, 12.09.2021 17:00

Social Studies, 12.09.2021 17:00

World Languages, 12.09.2021 17:00

Physics, 12.09.2021 17:10

English, 12.09.2021 17:10



English, 12.09.2021 17:10

Mathematics, 12.09.2021 17:10


 (At constant volume and number of moles)
(At constant volume and number of moles)
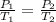
 = initial pressure of gas = 841 mm Hg
= initial pressure of gas = 841 mm Hg
 = final pressure of gas = ?
= final pressure of gas = ?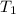 = initial temperature of gas =
= initial temperature of gas =
 = final temperature of gas =
= final temperature of gas = 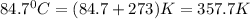
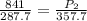
 ( 760 mm Hg = 1atm )
( 760 mm Hg = 1atm )



