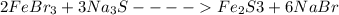Balance the following chemical
equation by providing the correct
coefficients.
[ ]FeBr3...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 23.06.2019 03:30, alecnewman2002
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 18:10, xojade
A1 forms when an acid is neutralized by a base. 1. salts can be neutral, or in solutions. salts of 2. strong acid–strong base reactions produce solutions with 3. water. salts formed from the neutralization of weak acids or weak 4. bases water. they produce solutions that are acidic or . basic. for example, the ph of a solution at the equivalence point is . greater than for a acid titration. solutions that resist changes in ph are called solutions. the buffer is the amount of acid or base that can be added to a buffer without changing the ph greatly.
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Social Studies, 25.08.2021 17:10


Mathematics, 25.08.2021 17:10


English, 25.08.2021 17:10

Computers and Technology, 25.08.2021 17:10